Heart tissue can regenerate — How Cold War nuclear tests led to major discovery
Study reveals extraordinary self-healing potential in advanced heart failure patients
December 23, 2024
TUCSON, Ariz. — For decades, medical science has insisted that the human heart cannot repair itself in any meaningful way. This dogma, as fundamental to cardiology as a heartbeat itself, is now being challenged by game-changing research that reveals our hearts may possess an extraordinary power of regeneration—provided they’re given the right conditions to heal.
The study, published in Circulation, offers potential new directions for treating heart failure, a condition that affects nearly 7 million U.S. adults and accounts for 14% of deaths annually, according to the Centers for Disease Control and Prevention.
Traditionally, the medical community has viewed the human heart as having minimal regenerative capabilities.
Unlike skeletal muscles that can heal after injury, cardiac muscle tissue has been thought to have very limited repair capacity.
“When a heart muscle is injured, it doesn’t grow back. We have nothing to reverse heart muscle loss,” says Dr. Hesham Sadek, director of the Sarver Heart Center at the University of Arizona College of Medicine – Tucson, in a statement.
It’s long believed that human heart muscle cells weren’t capable of regeneration, but a new study shows the “strongest evidence” yet, researchers say. (© appledesign – stock.adobe.com)
However, this new research, conducted by an international team of scientists, demonstrates that hearts supported by mechanical assist devices can achieve cellular renewal rates significantly higher than previously observed.
READ MORE: A Failing Heart Can Still Heal Itself: Study
The study examined tissue samples from 52 patients with advanced heart failure, including 28 who received left ventricular assist devices (LVADs) – mechanical pumps surgically implanted to help weakened hearts pump blood more effectively.
The research methodology centered on an innovative approach to tracking cell renewal. Using a technique that measures carbon-14 levels in cellular DNA – taking advantage of elevated atmospheric levels from Cold War nuclear testing – researchers could effectively date when cardiac cells were created.
This method provided unprecedented insight into the heart’s regenerative processes.
The findings revealed a stark contrast between different patient groups.
In healthy hearts, cardiac muscle cells (cardiomyocytes) naturally renew at approximately 0.5% per year.
However, in failing hearts, this renewal rate drops dramatically – to 0.03% in cases of non-ischemic cardiomyopathy (heart failure not caused by blocked arteries) and 0.01% in ischemic cardiomyopathy (heart failure from blocked arteries).
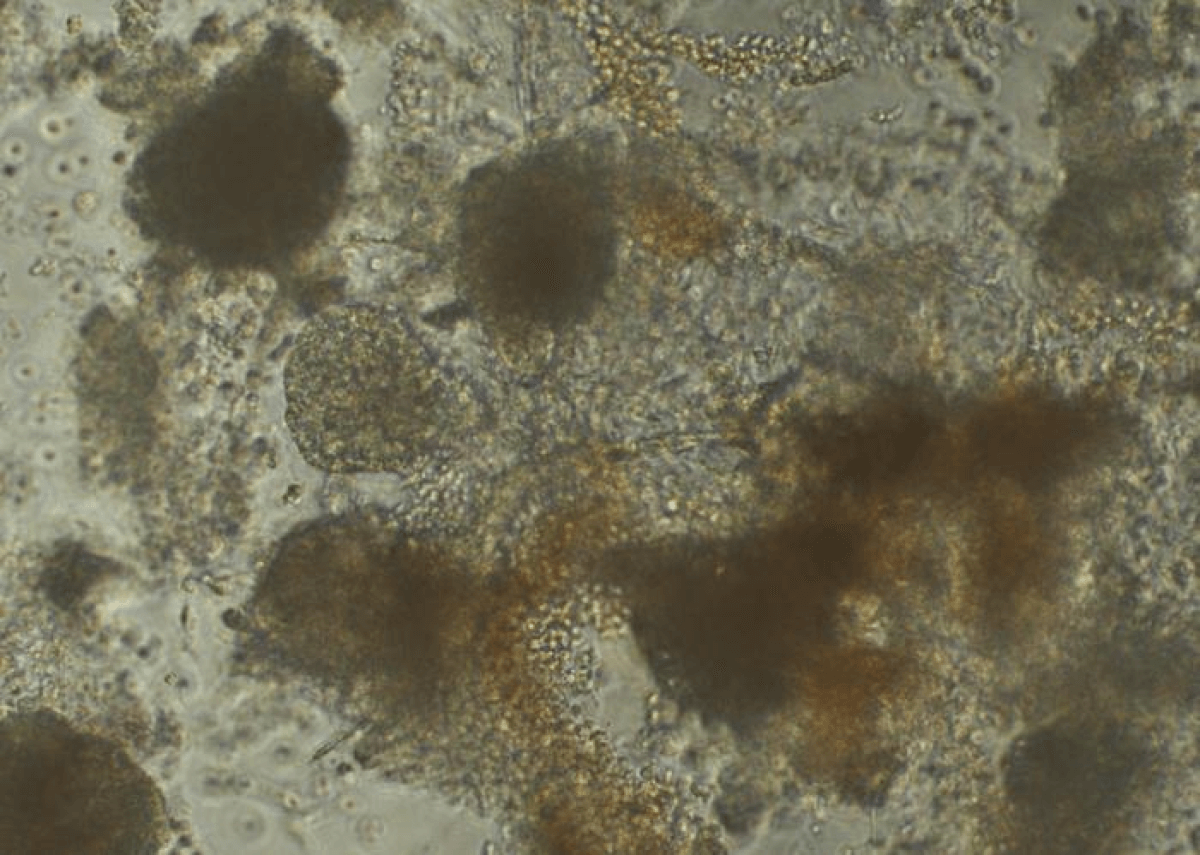
Cardiac muscle cells, or cardiomyocyte, under a microscope. (Credit: Trevor Atkeson and Madhavi Pandya, Garvan Institute)
The most significant finding emerged from patients who responded positively to LVAD support.
These “responders,” who showed improved cardiac function, demonstrated cardiomyocyte renewal rates more than six times higher than those seen in healthy hearts.
This observation provides what Dr. Sadek calls “the strongest evidence we have, so far, that human heart muscle cells can actually regenerate.”
The study builds upon previous research, including Dr. Sadek’s 2011 publication in Science showing that heart muscle cells actively divide during fetal development but cease shortly after birth to focus solely on pumping blood.
His 2014 research provided initial evidence of cell division in artificial heart patients, laying the groundwork for the current study.
The mechanism behind this increased regeneration may be linked to the unique way LVADs support heart function.
These devices effectively provide the cardiac muscle with periods of reduced workload by assisting with blood pumping, potentially creating conditions that enable regeneration.
This observation aligns with established knowledge about how other tissues in the body heal and regenerate when given adequate rest.
Scientists now hope to figure out why some patients are capable of having the advanced regenerative response and others aren’t. (© LIGHTFIELD STUDIOS – stock.adobe.com)
The research team found that in failing hearts, most cellular DNA synthesis is directed toward making existing cells larger or more complex through processes called polyploidization and multinucleation, rather than creating new cells.
However, in LVAD patients who showed improvement, a significant portion of DNA synthesis was dedicated to generating entirely new cardiac muscle cells – a more beneficial form of cardiac adaptation.
Approximately 25% of LVAD patients demonstrate this enhanced regenerative response, raising important questions about why some patients respond while others do not.
Understanding these differences could be crucial for developing new therapeutic approaches.
“The exciting part now is to determine how we can make everyone a responder,” says Sadek.
The implications of this research are particularly promising because LVADs are already an established treatment option.
As Dr. Sadek points out, “The beauty of this is that a mechanical heart is not a therapy we hope to deliver to our patients in the future – these devices are tried and true, and we’ve been using them for years.”
Paper Summary
Methodology
The study employed several sophisticated techniques to examine heart regeneration. Researchers collected cardiac tissue samples during transplant procedures and analyzed them using carbon-14 dating of DNA, flow cytometry for cell isolation, and advanced imaging techniques.
Mathematical modeling helped calculate renewal rates and determine how different cellular processes contributed to DNA synthesis.
Results
The study documented the decline in regeneration rates from 0.5% per year in healthy hearts to 0.01-0.03% in failing hearts.
In LVAD responders, regeneration increased to approximately 3.1% per year, accompanied by beneficial changes in cell structure and function.
Limitations
Several important limitations should be noted.
The study was observational in nature, meaning it cannot definitively establish cause-and-effect relationships.
The relatively small sample size and retrospective nature of carbon-14 dating also limit certain conclusions.
Additionally, the researchers could not directly observe cell creation in real-time, relying instead on mathematical modeling to interpret their findings.
Discussion and Takeaways
This research represents a significant advance in understanding cardiac regeneration, but it’s important to note that while the findings are promising, they don’t yet translate directly into a cure for heart failure.
The study does, however, open new avenues for research into why some patients respond better to LVAD therapy and how this regenerative capacity might be enhanced in all patients.
The discovery that the human heart retains this regenerative potential, even if dormant, challenges long-held assumptions about cardiac healing and may lead to new therapeutic strategies.
Future research will need to focus on understanding the molecular mechanisms that enable this regeneration and how they might be activated in more patients.
Funding and Disclosures
This international collaboration was supported by multiple institutions, including funding from the Leducq Foundation Transatlantic Networks of Excellence Program, which specifically facilitates collaboration between American and European investigators.
Key contributions came from the University of Utah Health and School of Medicine and the Karolinska Institute in Stockholm, among others.
The researchers declared no conflicts of interest.